Health Effects: Ozone, the main component of smog, is the most widespread air pollutant. It is a powerful respiratory irritant at levels found in much of the nation during warm weather. The U.S. Environmental Protection Agency reports that 107 million Americans live in areas that violate health standards for ozone. Symptoms include shortness of breath, pain during deep breaths, wheezing and coughing.
Ozone exposure has been linked to increased hospital admissions and emergency room visits for asthma and other respiratory problems. It can also reduce the body's resistance to infection. Long-term, repeated exposure to high levels of ozone may lead to large reductions in lung function, inflammation of the lung lining and more frequent and severe respiratory discomfort. The American Lung Association likens ozone exposure to a "sunburn on the lung." Recent studies have also linked ozone to premature death, though research continues on this topic.
Ozone is especially dangerous for children, the elderly, people with chronic lung and heart disease -- even healthy people who exercise outdoors.
Children are at particular risk because their lungs are still growing and developing. They breathe more rapidly and more deeply than adults do, so a greater doze of air pollution may be delivered to their lungs. Children also spend significantly more time outdoors, especially in summer when ozone levels are highest.
Sources: Ozone is not emitted directly into the air, but is formed by the reaction of volatile organic compounds (VOCs) and nitrogen oxides (NOx ) in the presence of heat and sunlight. VOCs are emitted by a variety of sources, including motor vehicles, chemical plants, refineries, and other factories. NOx is emitted by motor vehicles, electric power plants, and other combustion sources. Ozone can be transported into an area from pollution sources found hundreds of miles upwind.

Ozone is great, high up in the atmosphere as it reflects heat and other radiates. But closer to earth, ozone is an air pollutant that can be harmful to humans.
When ground level ozone is created, it hangs around in the layer of air near the ground. This is typically a band of air from 0 to 10 miles high; where it affects and reacts with everything it comes in contact with.
Ozone is a colorless odorless gas made of oxygen with three oxygen atoms joined together with the chemical designation O3. Ozone is is a highly reactive substance and is ready to react with whatever it meets upon contact.
| Ground-Level Ozone (O3) | Health Effects (not all of these are noticeable) | Particulate Matter |
| x | Coughing, irritation of the airways, discomfort in the chest or when breathing | |
| x | Premature aging of the lungs | |
| x | Faster or more shallow breathing | x |
| x | Aggravation of asthma, emphysema, and other respiratory diseases | x |
| x | Increased risk of respiratory infections | x |
| Premature death (primarily among older adults and those with existing heart and lung disease) | x |
On summer days, the sun can easily scorch your skin, damaging layers of tissue. Your skin turns bright red and becomes very painful. Like sunburn, ozone can sear your lungs and airways causing tissue to redden and swell. Within a few days, the damaged cells are replaced and the old cells are shed in much the same way that skin peels after a sunburn. However, just like sunburn, if this kind of damage occurs repeatedly, it may start to cause permanent damage to your lungs.
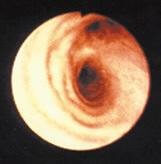
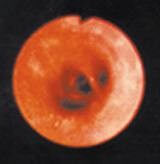
These photos show a healthy lung air way (left) and an inflamed lung air way (right).

A chemical reaction creates ground-level ozone from volatile organic compounds (VOCs) and nitrogen oxides (NOx). VOCs and NOx are produced in the morning by cars and trucks. They remain in the air until the afternoon, when the sun combines them into ozone.
This happens because air is mostly nitrogen and oxygen. In air, most molecules of both nitrogen and oxygen contain two atoms. The combustion of fossil fuels reach such high temperatures that the nitrogen and oxygen in the air combine to form nitric oxide. This nitric oxide quickly combines with more oxygen in the air to form nitrogen dioxide.
Normally in warm, sunny air, the nitrogen dioxide breaks down into a molecule of nitric oxide and an atom of oxygen. The atom of oxygen combines with a molecule of oxygen to form another form of oxygen, this form of oxygen has 3 oxygen atoms in its molecule and is called ozone. The nitric oxide reacts with the ozone to form nitrogen dioxide and an ordinary oxygen molecule and we are back where we started.
This cycle can be broken by the other substances we add to the atmosphere. Evaporative fumes from fuels, solvents from drying paint and from drying glue all add something to the air. Substances like these are called VOC�s, short for "volatile organic compounds." When nitrogen dioxide is broken down by sunlight, the VOC�s combine with the nitric oxide and change it back to nitrogen dioxide. Ozone still forms as usual, but there is much less nitric oxide to remove it, so ozone accumulates and it's concentration in the air rises.
Morning rush hour traffic is a major source of NOx and VOC�s. Adding to this effect, is the fact that carbon monoxide can behave as a low reactive VOC in the atmosphere. Vehicular carbon monoxide emissions acting a lot like VOC's in the air during peak rush hour times will be increasing the atmospheres ozone generating capacity.
Ozone is typically produced in greater amounts on hot, sunny days when there is little wind. On a clear day, ozone levels can continue to rise all day long, and then decrease rapidly after sunset. Since heat, sunlight and the ingredient gasses each usually increase during the day, ozone formation also increases during daylight hours. When the sun goes down, there is no energy for ozone formation and fewer ingredient gases available, so ozone levels drop. Ozone levels are usually highest during the hottest part of the afternoon. Ozone is so highly reactive that it quickly reverts to other forms with a half life of around 30 minutes, so during the night time ozone levels usually dissipate quickly.
One thing to keep in mind about NOx reductions is that this cycle is a balancing reaction and tampering with either side of the equation can get you into trouble.
There is an an effect called the "weekend effect" that sees increases in ground level ozone and might be due to the fact that there is LESS NOx in the air. The weekend ozone effect would suggest lowering NOx emissions may actually make ground level ozone worse.